Medical Devices & Instruments
Experience faster and more cost-effective medical device production.
Request PricingPrecision Micro provides medical device designers with a rapid, clean and cost-efficient ISO-accredited chemical etching service, unrestricted by the limitations of conventional machining methods.
Traditional medical device machining
Medical devices are vital in healthcare, saving lives and improving patient outcomes. Stamping and laser cutting have been primary manufacturing technologies used to produce metal parts due to their precision and efficiency. However, these methods have limitations, such as material stress and burrs, which can affect quality, cleanliness and repeatability. Addressing these challenges is crucial for producing high-quality medical components.
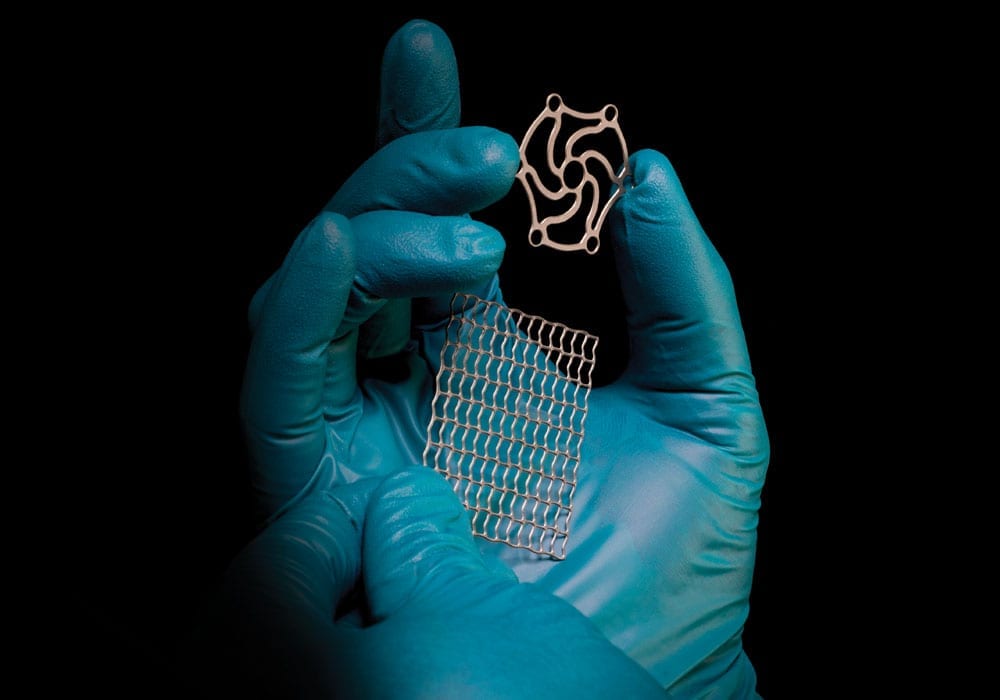
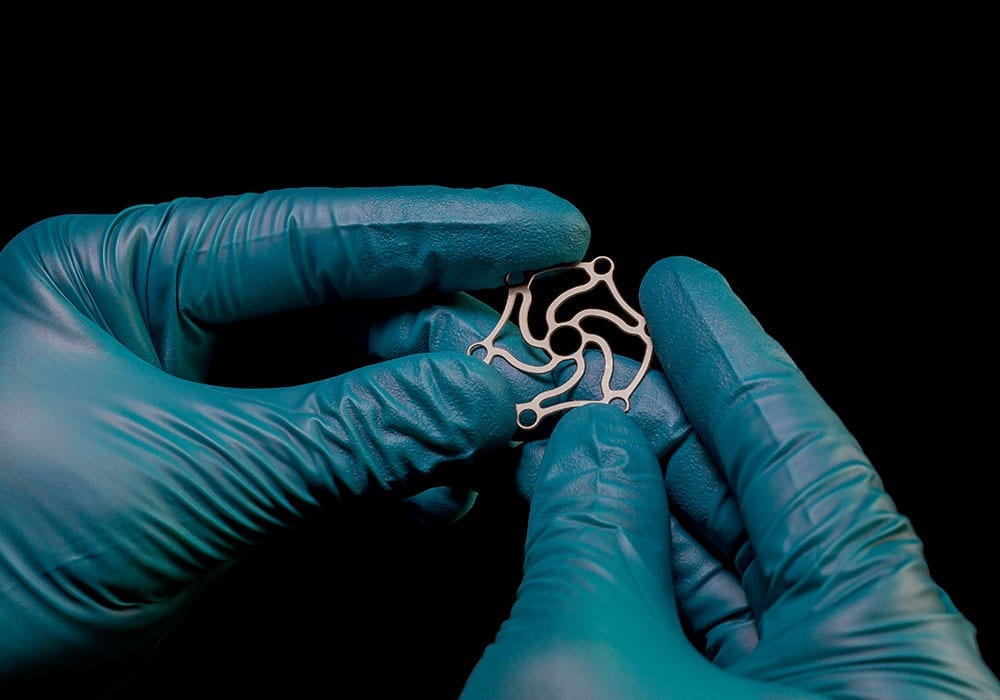
Precision-etched medical components & devices
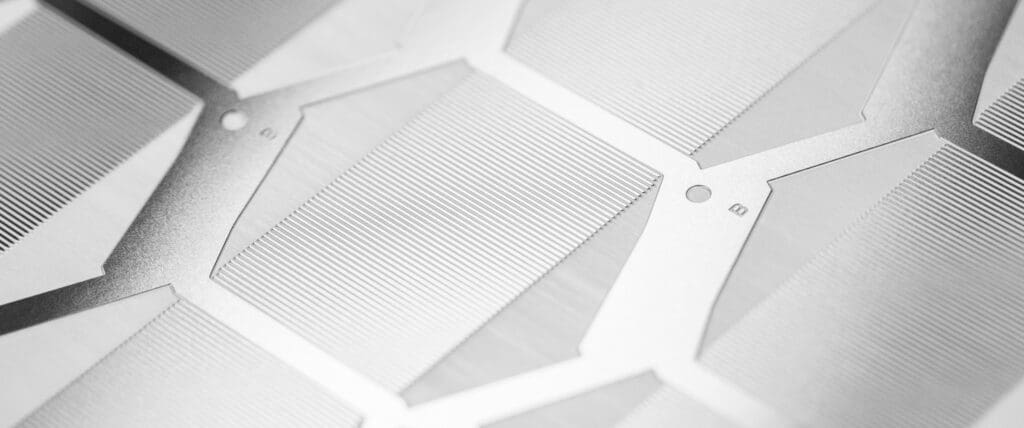
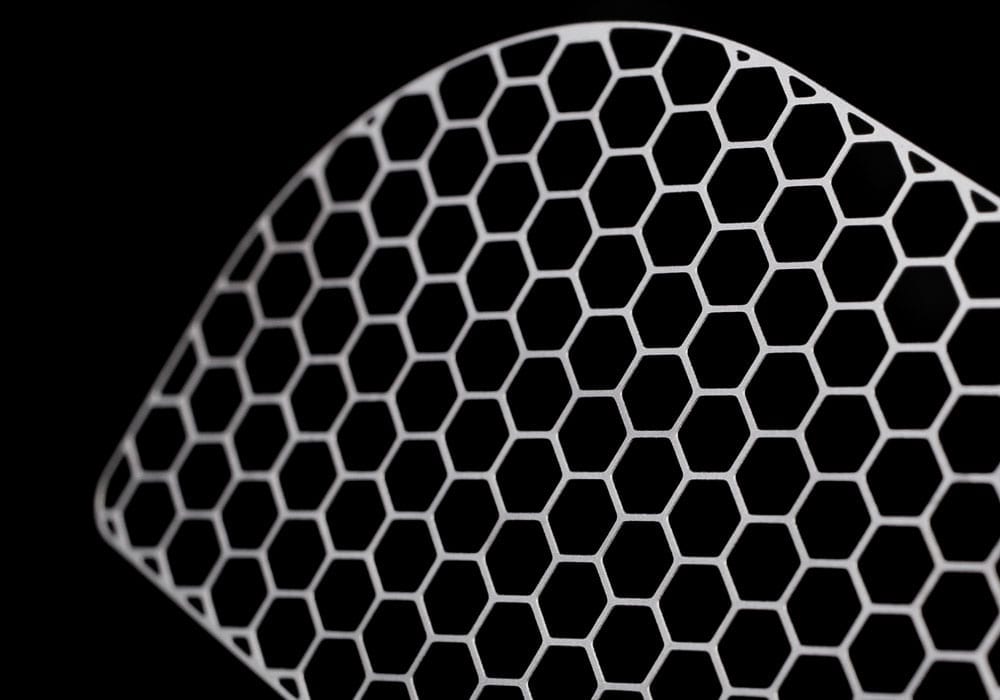
Chemical etching is an ideal alternative to stamping and laser cutting, effectively overcoming the limitations often associated with these traditional machining techniques. Chemical etchants are employed to selectively dissolve material from a metal sheet, eliminating the risk of any adverse part defects and guaranteeing 100% burr and stress-free medical components.
Economical, fast & accurate
Our digital tooling enables rapid prototyping and seamless design modifications, leading to cost and lead time reduction. Engineers can freely design without cost penalties for complex geometries, and the process can achieve feature sizes as small as 0.1mm with an accuracy of ±0.020mm.
Benefits
Low cost digital tooling

Rapid prototyping

No cost penalty for complex designs
100% burr and stress-free
Versatile component machining
Photo etching is a versatile machining process that accommodates various metal types and grades. This adaptability allows medical device designers to choose the most suitable metal for each specific component, ranging from ultra-hard stainless steels for surgical instruments to corrosion-resistant, biocompatible materials for implantable devices.
Chemical Etching Whitepaper
Learn how chemical etching can overcome the limitations of traditional sheet metal machining technologies.
Download